The Q-Symbio study

Q-symbio is a randomized, placebo-controlled, double-blinded study,
begun in 2003 under the direction of Associate Professor Svend Aage
Mortensen (picture) from the Copenhagen University Hospital. The study
involved 420 patients with severe heart failure (NYHA* Class III or
IV) from nine different countries. The participants were randomly
chosen to receive either soft gelatin capsules with Q10 as ubiquinone
each day or identical-looking placebo capsules. The participants in
the two groups were then followed for 2 years.
The aim of the
study was to investigate how much time would elapse until the
participants in the two groups again encountered heart problems,
meaning unplanned hospitalizations because of heart failure, fatal
heart attacks, a need for cardiac transplantation, or a need for a
mechanical assist pump (a heart pump).
Largest
placebo-controlled study
If you calculate the study's
patient-years, the Q-symbio clinical trial is actually the largest
placebo-controlled study of coenzyme Q10 for heart failure in the
world to date. The study and its groundbreaking results have also
aroused great interest and curiosity among medical doctors and
scientists in many countries and especially in the U.S., where Svend
Aage Mortensen has given several presentations on the results. More
interest in coenzyme Q10 will be generated by the publication of the
study results..
* The NYHA classification is an official ranking system used
globally that divides heart failure patients into four individual
categories depending on the severity of their symptoms.
What
are the research results?
The study showed
that patients in the Q10 group had a 43% decrease in cardiovascular
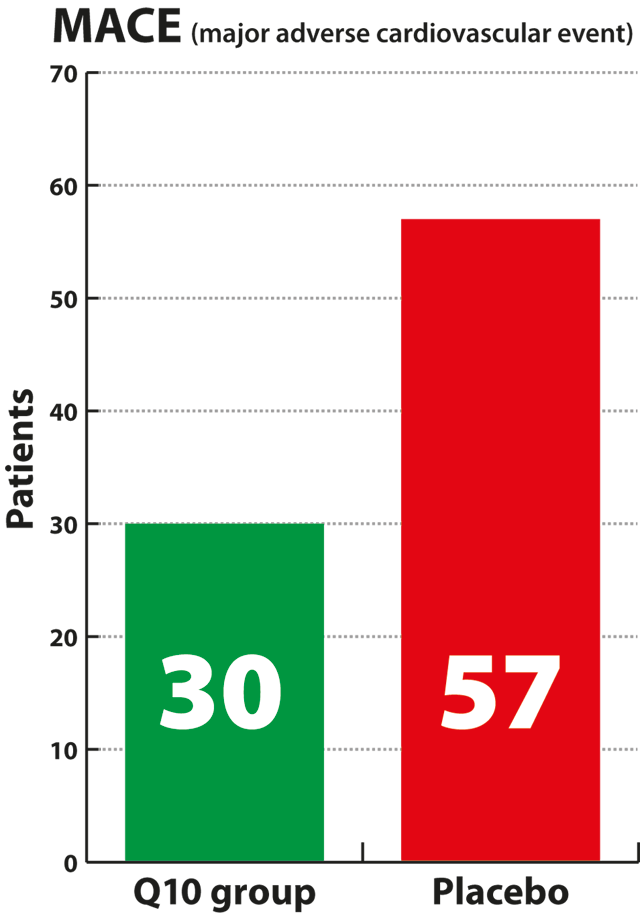
death compared to the placebo group. Only 15% of the patients in the
Q10 group experienced serious heart events, called MACE events,
compared with 26% of the patients who received the placebo,
corresponding to a 43% relative reduction, which was a statistically
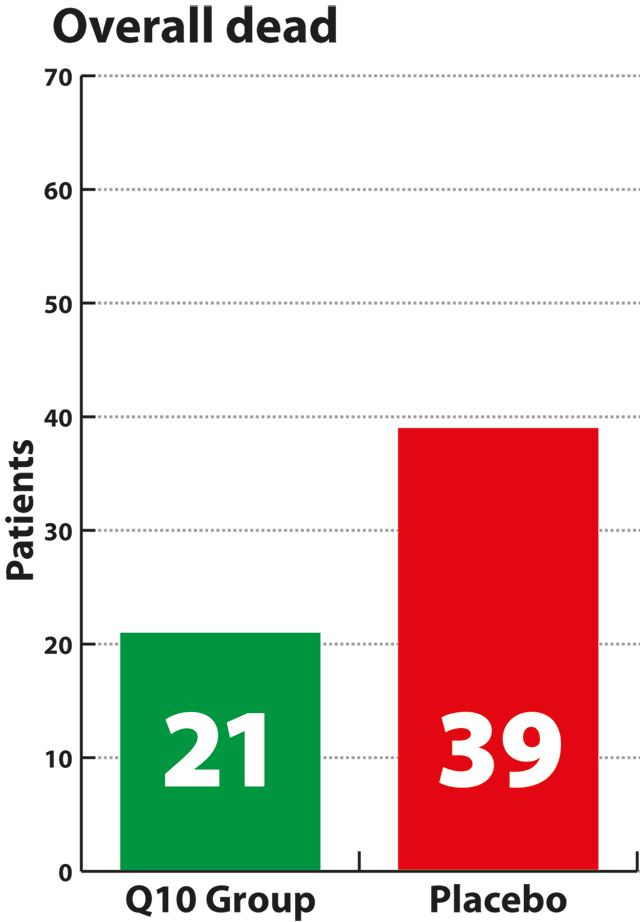
significant result. Supplementation with Q10 also significantly
reduced the risk of death from all causes by 42% compared to the
placebo group. The number of unplanned hospitalizations was 8% in the
Q10 group against 14% in the placebo group, which was also a
statistically significant difference. Moreover, there were
significantly more of the Q10-treated patients who improved one or
more NYHA class than in the group receiving placebo (58% versus 45%).
One patient in the Q10 group went from NYHA IV to NYHA I during his
treatment. In addition, a biological marker for heart disease called
NT-proBNP was slightly improved in the Q10 group, and the Q10 group
also experienced fewer side effects.
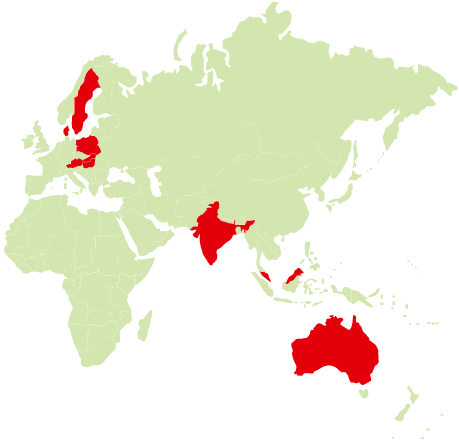
The trial participants came from the countries marked above
“The study gives rise to seriously considering adding to the guidelines for the treatment of heart failure patients. I predict that Q10 supplements eventually will become common treatment worldwide for these patients.”
Chief Physician Dr. Steen Stender, Clinical Biochemistry Department, Gentofte Hospital, Quoted in Jyllandsposten. 2014, Oct.19, in response to the Q-symbio study.
“CoQ10 is the first new medication to improve survival in chronic heart failure, and it should be added to standard therapy."
Assoc. Prof. Dr. Svend Aage Mortensen, Head of the Q-Symbio study.
New data from the Q-Symbio study
At a Q10 conference at Columbia University in New York 2018, Dr. Svend Aage Mortensen's daughter, biochemist Anne Louise Mortensen, presented new data from the European subgroup of the patients in the Q-Symbio study. This new analysis included 231 patients from Denmark, Sweden, Austria, Slovakia, Poland and Hungary. The Q10 group comprised 108 patients who received (Myoqinon) 100 mg Q10 x 3 daily. The placebo group with similar characteristics represented 123 patients.
Anne Louise Mortensen's analysis confirmed a similarly good effect of Q10 on the European part of the patient group. For example:
| Reduction of | EU-patients | All patients |
|---|---|---|
| Severe cardiac events (MACE) | 67% | 43% |
| Cardiovascular mortality | 53% | 43% |
| Death from any cause | 55% | 42% |
Why were there significantly better results in the European subgroup?
- Probably because these patients formed a more homogeneous group
- Their serum Q10 has been higher throughout the investigation period
- There may have been better compliance (patients have been better taking their medication)?
- Another diet?
- Genetic differences?
References
Egwim C, et al. Global variations in Patient populations and Outcomes in Heart Failure Clinical Trials. Curr. Heart Fail. Rep. 14, 30-39 (2017).
Mortensen SA, et al. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: results from Q-SYMBIO: a randomized double-blind trial. JACC. Heart Fail. 2,641-9 (2014).








